Blog Standard
Advancing Cancer Diagnostics with Precision CTC Analysis
The field of cancer diagnostics is rapidly evolving, with a growing emphasis on technologies that can detect and analyze rare cells in the bloodstream, such as circulating tumor cells (CTCs). These cells, which are shed from tumors and circulate in the blood, hold immense potential for non-invasive cancer detection and treatment monitoring. However, their extreme rarity—often just one CTC per 10 billion red blood cells—presents significant challenges for accurate detection. To address this, innovative solutions like RareCyte’s AccuCyte and CyteFinder Instrument, combined with Cellenion’s cellenONE for CTC analysis, are revolutionizing rare cell analysis technology by enabling precise and reproducible validation of CTC assays.

RarecytCye teFinder® II
A Novel Approach to CTC Validation
The rarity of CTCs demands technologies that combine high sensitivity with robust reproducibility. RareCyte, a leader in circulating tumor cell analysis, has developed the AccuCyte-CyteFinder system, which offers an end-to-end workflow for CTC enumeration and biomarker expression analysis in cancers such as breast cancer, prostate cancer, and lung cancer. To ensure the accuracy required for clinical trials and phases, rigorous assay validation is critical. This involves creating surrogate blood samples with known quantities of model CTCs (mCTCs) at levels close to the assay’s limit of detection.
Traditional methods like serial dilution or flow cytometry often fall short in delivering the precision needed for such low cell counts. To overcome this, RareCyte partnered with Cellenion to leverage the circulating tumor cell spike-in method using the cellenONE instrument. The cellenONE is a single-cell isolation and dispensing technology that uses automated image recognition to ensure each dispensed droplet contains exactly one cell. Originally designed for single-cell analyses and cell line development, this cell dispenser has been adapted for CTC assay validation, offering unmatched accuracy and precision.
The Validation Workflow
The collaboration between RareCyte and Cellenion resulted in a tailored workflow for CTC assay validation, as outlined in Figure 1. A customized sample deck was designed for the cellenONE, enabling precise deposition of cells onto microscope slides or into RareCyte’s AccuCyte Blood Collection Tubes (BCTs) containing 7.5 ml of whole blood. The cellenONE was programmed to dispense 100 cells per slide or 5–8 cells per BCT. These Cellenion samples were then processed using the AccuCyte system, stained with the RarePlex Enumeration Panel Kit, and analyzed with the CyteFinder Instrument to count CTCs.
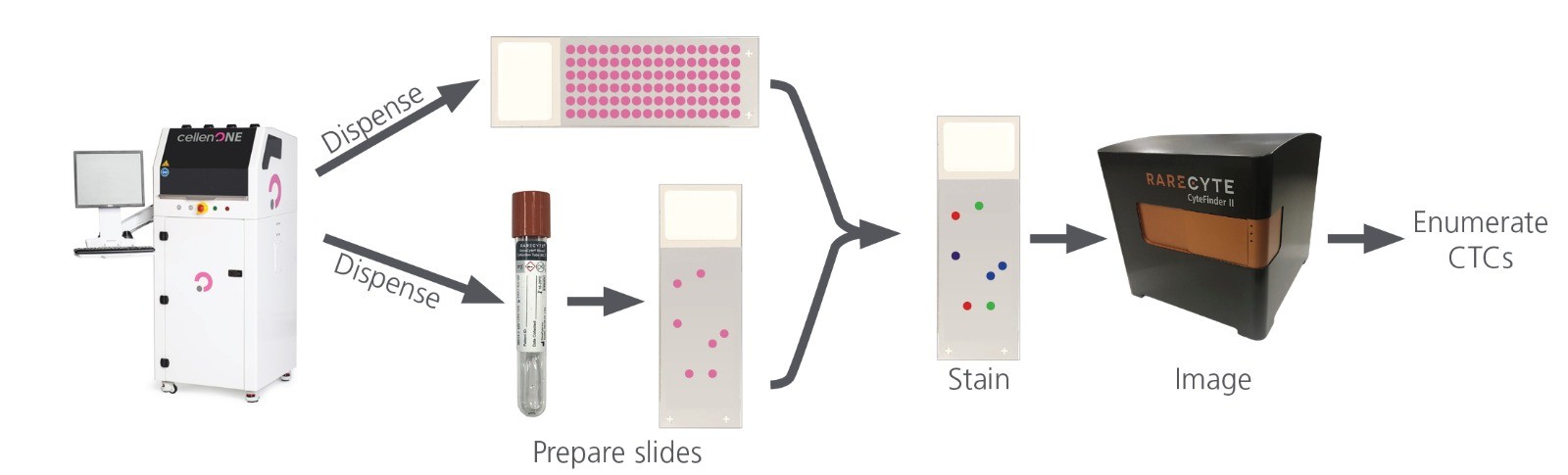
Figure 1 5-step CTC enumeration validation workflow. Dispense cells with cellenONE onto slides and into BCT; process BCT to slides with AccuCyte system; stain slides with RarePlex® Enumeration Panel Kit; image and analyze with CyteFinder Instrument to count CTCs.
This five-step workflow ensures that rarecyte circulating tumor cell assays are validated with a high degree of standardization, critical for clinical applications. The ability to deposit precise numbers of mCTCs, such as HAP1 cell and SW900 cell lines, into blood samples allows researchers to mimic the clinical scarcity of CTCs, making validation studies both accurate and reproducible.
Impressive Results with High Recovery Rates
The results of this circulating tumor cell spike-in method are remarkable, as shown in Tables 1 and 2. Table 1 demonstrates the accuracy of the cellenONE system when printing 100 mCTCs directly onto slides. Across eight replicates, a total of 800 cells were dispensed, with 797 recovered, yielding an impressive recovery rate of 99%. This near-perfect accuracy confirms the reliability of the cellenONE for high-throughput applications.
| Replicate | Print # | mCTC Count |
|---|---|---|
| 1 | 100 | 100 |
| 2 | 100 | 100 |
| 3 | 100 | 100 |
| 4 | 100 | 100 |
| 5 | 100 | 99 |
| 6 | 100 | 99 |
| 7 | 100 | 100 |
| 8 | 100 | 99 |
| Total | 800 | 797 |
| Recovery | 0.99 |
Table 1 mCTC count for slides printed with 100 mCTCs.
| Replicate | Spike-in # | mCTC Count |
|---|---|---|
| 1 | 8 | 8 |
| 2 | 5 | 5 |
| 3 | 5 | 5 |
| 4 | 5 | 4 |
| 5 | 5 | 5 |
| 6 | 5 | 4 |
| 7 | 5 | 5 |
| 8 | 5 | 5 |
| 9 | 6 | 6 |
| Total | 49 | 47 |
| Recovery | 0.96 |
Table 2 mCTC count from deposition of 5-8 cells per BCT.
Table 2 further highlights the precision of the system when spiking 5–8 mCTCs into BCTs. Across nine replicates, 49 cells were spiked, with 47 recovered, resulting in a 96% recovery rate. These high recovery rates underscore the effectiveness of combining RareCyte’s AccuCyte and cellenONE technologies for preparing Cellenion samples that closely mimic clinical conditions.
Figure 2 provides visual confirmation of the assay’s specificity. It shows images of mCTCs from HAP1 cell and SW900 cell spike-in assays, identified by the presence of a nucleus, localization of Cytokeratin (CK) to the cytoplasm or Epithelial Cell Adhesion Molecule (EpCAM) to the cell membrane, and the absence of CD45. These markers ensure accurate identification of CTCs, even at low concentrations. Figure 3 further illustrates the precision of the cellenONE, showcasing an image of 100 mCTCs printed onto a slide, a testament to the system’s ability to handle single-cell deposition with consistency.
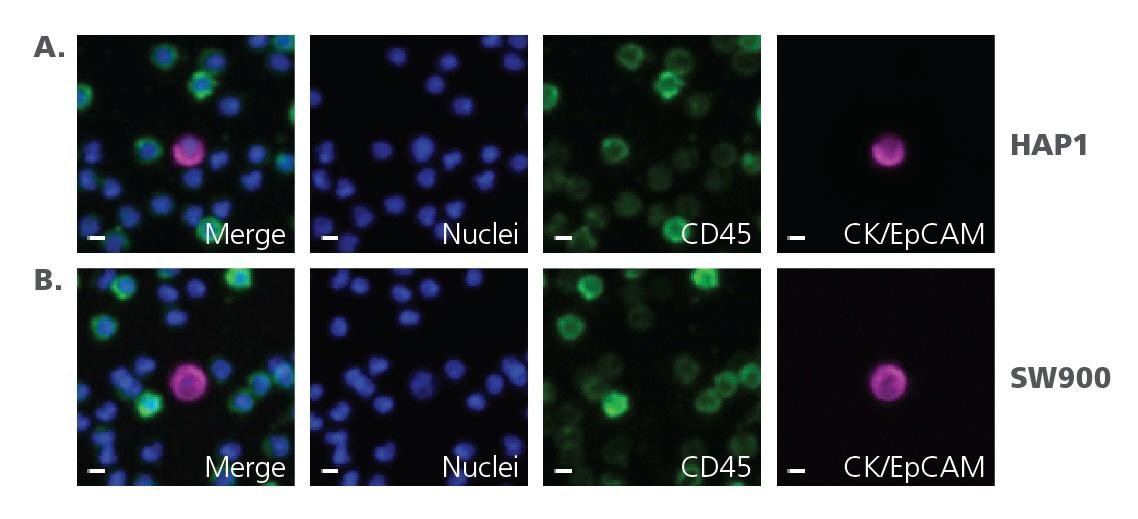
Figure 2 mCTC images from cellenONE spike-in assay. CTCs are identified by the presence of a nucleus, localization of Cytokeratin (CK) to the cytoplasm or Epithelial Cell Adhesion Molecule (EpCAM) to the cell membrane, and absence of CD45 localization. A. HAP-1 mCTC spike-in assay. B. SW900 mCTC spike-in assay. Scale bar represents 5 μm.
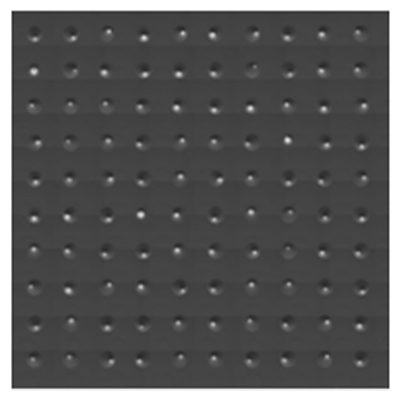
Figure 3 Image of 100 mCTCs printed to a slide.
Transforming Cancer Diagnostics
The integration of cellenONE into RareCyte’s validation workflow has set a new standard for circulating tumor cell analysis. Unlike flow cytometry or serial dilution, which struggle with low cell counts, the cellenONE delivers unparalleled accuracy and reproducibility. This makes it an essential tool for rare cell analysis technology, particularly in the context of clinical trials and phases where precision is non-negotiable.
By enabling the creation of standardized samples with known mCTC counts, the cellenONE ensures that RareCyte’s CTC assays meet the stringent requirements of cancer diagnostics. This technology not only enhances the reliability of CTC enumeration but also supports biomarker expression analysis, paving the way for personalized treatment strategies in breast cancer, prostate cancer, and lung cancer.
Conclusion
The work at Cardiff University highlights the transformative potential of chemical synthesis in addressing environmental challenges. By leveraging the Radleys Reactor-Ready system for polymer scale production and developing recyclable, glowing polymers, researchers are redefining the possibilities in the plastics recycling industry. These materials science innovations not only solve critical recycling challenges but also open new avenues for creating advanced, sustainable materials. As this technology scales, it promises to reshape how we produce, use, and recycle polymers, fostering a more sustainable and innovative future.
Inkarp Instruments stands as a trusted distributor and service provider of RareCyte’s advanced scientific solutions in India, delivering cutting-edge technology tailored to the dynamic needs of modern research. Committed to excellence, we empower researchers across the nation with innovative tools and unparalleled expertise, fostering breakthroughs and driving progress in scientific discovery.
References: RareCyte

