Blog Standard

Analytical Validation of an Immunofluorescence Assay for ARv7 Protein Expression on Circulating Tumor Cells Using the RareCyte Platform
In the realm of precision oncology, the characterization of circulating tumor cells (CTCs) through non-invasive methods like liquid biopsy has gained significant traction. These advancements offer real-time insights into tumor heterogeneity, therapy resistance, and potential biomarker identification in various cancers, particularly prostate cancer. A growing area of focus is the detection of the androgen receptor splice variant ARv7, which has been associated with resistance to anti androgen therapies. This document outlines the analytical validation of an immunofluorescence assay developed to detect ARv7 protein expression on CTCs using the RareCyte Platform, thus providing a powerful tool for assessing treatment efficacy and guiding therapeutic decisions.
Study Background
CTCs represent a valuable diagnostic resource by enabling blood sample analysis for assessing drug target expression and disease progression without invasive procedures. The presence of ARv7 in tumor cells derived from prostate cancer patients is linked to poor response to second-generation anti androgen therapies. This study aimed to validate a robust immunofluorescence-based assay to detect and quantify ARv7 expression using the RareCyte Platform, with the potential to refine treatment strategies and monitor patient response through liquid biopsy.
Methods
The study utilized blood samples from healthy donors, spiked with prostate cancer cell lines representing a range of ARv7 expression levels: 22RV1 (high), LNCAP (low), and BT-474 (negative). Sample preparation was executed using the AccuCyte Sample Preparation System.
Staining was conducted with an automated slide staining system, using the RarePlex ARv7 CTC Panel Kit. This panel incorporates:
- A nuclear dye
- Anti-CD45 (to exclude leukocytes)
- Antibodies to cytokeratin and epithelial cell adhesion molecule (EpCAM)
- An ARv7-specific antibody
Slides were then imaged using the CyteFinder Instrument, and CTC detection was performed via a machine learning-based algorithm followed by expert review. For quantification, mean fluorescence intensity (MFI) was used as a measure of ARv7 protein levels, providing an objective readout of expression.

Rarecyte CyteFinder II
Results
The study successfully established an ARv7 MFI threshold that reliably distinguished ARv7-positive from ARv7-negative cells. The assay identified 83% of 22RV1 cells as positive and 98% of BT-474 cells as negative, demonstrating an overall accuracy of 90%. Importantly, ARv7-positive staining in clinical prostate cancer samples was appropriately localized to the nucleus, confirming the assay’s biological relevance.
When compared with a standard CTC detection assay, recovery rates using the ARv7 assay were at least equivalent, validating its efficiency. Furthermore, the ARv7 assay allowed for precise single cell analysis, contributing to a deeper understanding of tumor heterogeneity.

Figure 1. The RareCyte ARv7 CTC assay workflow. Blood was collected into AccuCyte Blood Collection Tubes. Nucleated blood cells were processed to slides using the density-based AccuCyte Sample Preparation System. Slides were stained with the RarePlex ARv7 CTC Panel Kit using the Leica® BOND RX automated slide staining system. Slides were scanned using the CyteFinder II Instrument and images were analyzed using CyteMapper® software and analysis tools. CTCs were analyzed by a trained reviewer and CTC ARv7 status was determined with a fluorescence intensity threshold.
| ARv7− | ARv7+ | ||
|---|---|---|---|
| BT-474 | 22Rv1 | LNCaP | |
| Test Positive (MFI > 100) | 24 | 832 | 245 |
| Test Negative (MFI ≤ 100) | 976 | 168 | 755 |
| Specificity | 0.976 | ||
| Sensitivity | 0.832 | 0.245 | |
| Accuracy | 0.904 | 0.611 | |
Table 1. Summary of Analytic Validation results. Sensitivity, specificity, and accuracy calculated on ARv7 positive and negative cell lines using an MFI cutoff of 100.
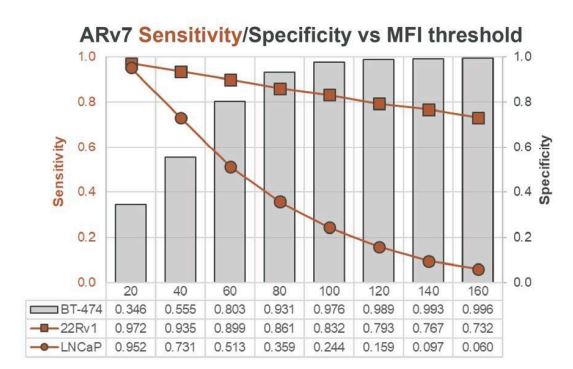
Figure 2. Sensitivity/specificity of ARv7 detection on cell lines. Method for determining ARv7 MFI intensity threshold. Sensitivity is graphed in two curves, one for cell line 22RV1 (ARv7-high) and one for LNCaP (ARv7-low). Bars and right axis values indicate Specificity for the biomarker-negative cell line BT-474. An MFI cutoff of 100 was selected that achieved a minimum Specificity value of 0.9. A cell with an MFI value greater than the 100 MFI threshold constitutes a positive test with the ARv7 assay for validation studies.
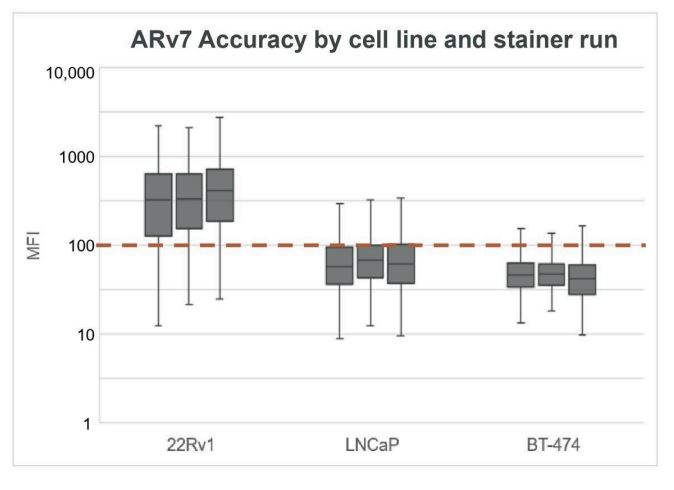
Figure 3. Accuracy of ARv7 detection on cell lines by stainer run. Distribution of ARv7 MFI for stainer run 1 (left), 2 (center), and 3 (right) for each cell line. Threshold dotted line at MFI=100 is used to determine biomarker expression status on a per-cell basis.
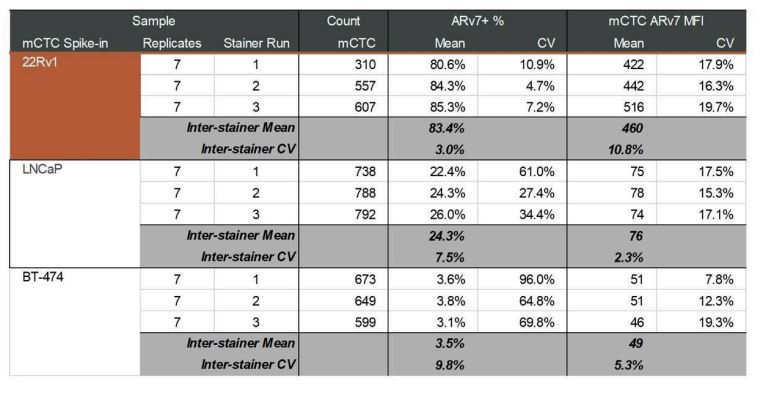
Table 2. Inter-stainer run mean and CV. ARv7 MFI and CV shown for each cell type and stainer run. Each run consisted of 7 slide replicates. ARv7 percent positivity was determined using an MFI cutoff of 100.
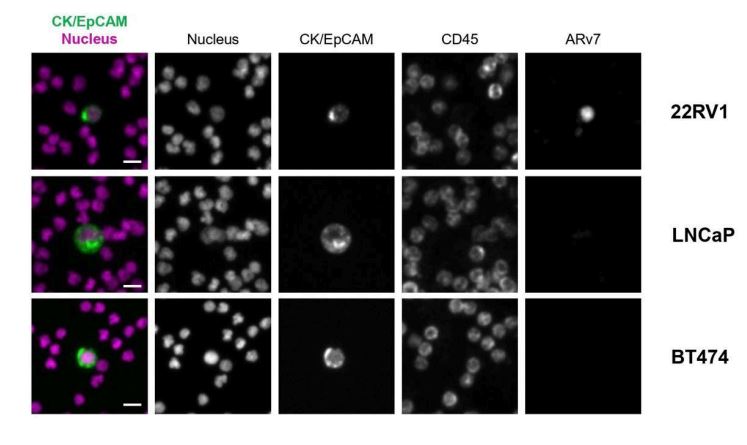
Figure 4. mCTC stained for ARv7. Representative images of mCTC identified with the ARv7 Panel Kit. Scale bars represent 5 μm.
| Patient | Indication | Gold Standard | ARv7 Assay |
|---|---|---|---|
| 1 | Prostate | 1 | 1 |
| 2 | Prostate | 0 | 0 |
| 3 | Prostate | 3 | 4 |
| Total | 4 | 5 |
Table 3. Clinical comparison results. Results of a small clinical comparison study comparing the number of CTCs counted using the ARv7 Staining Kit and a validated CTC detection assay (Gold Standard).
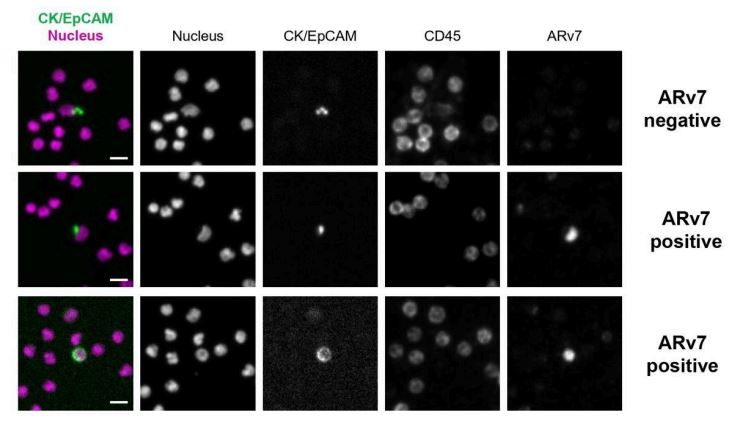
Figure 5. Clinical CTC stained for ARv7. Representative images of clinical CTCs obtained by staining slides from prostate cancer patients with the ARv7 Staining Kit, with status indicated, and with ARv7 MFI shown on respective images. Scale bars represent 5 μm.
Conclusion
This analytical validation confirms that the ARv7 immunofluorescence assay is a sensitive, specific, and reproducible method for detecting ARv7 expression in CTCs from blood samples. The assay performed robustly in differentiating ARv7-positive from negative cells, and its performance was on par with existing CTC detection technologies.
In clinical samples, both ARv7-positive and ARv7-negative CTCs were successfully identified, demonstrating its potential utility in early and advanced clinical study stages. However, a larger cohort is necessary to establish a definitive clinical threshold for ARv7 positivity.
Crucially, this assay facilitates androgen receptor status monitoring in a non-invasive manner and can serve as a predictive tool for therapeutic response. As ARv7 has been implicated in resistance to anti androgen therapies, the ability to track its expression through liquid biopsy holds promise for personalized treatment planning.
The ARv7 assay is also compatible with the RarePlex 488 Developer Kit, allowing researchers to incorporate an additional biomarker for multiplexing, thereby broadening the scope of fluorescence intensity-based analysis in immunofluorescence assays.
Inkarp Instruments is India’s leading distributor and trusted service partner for Rarecyte products. Driven by a commitment to innovation and excellence, Inkarp delivers state-of-the-art scientific equipment and dependable support to researchers across the nation.
Reference: Rarecyte

