Blog Standard

Revolutionizing Sample Preparation in Analytical Laboratories
In pharmaceutical analysis, precision and error reduction are paramount for high-quality results. Traditional volumetric methods often introduce variability, leading to Out of Specification (OOS) results that disrupt analytical laboratory workflows. Gravimetric sample preparation offers a transformative solution, enhancing weighing accuracy in labs, reducing laboratory errors, and improving efficiency. This blog explores how the gravimetric method addresses these challenges, with insights from key figures and tables in the original document.
Understanding Gravimetric Sample Preparation
Gravimetric sample preparation involves weighing both the solid sample and the solvent to achieve precise concentrations, unlike the volumetric method, which relies on less accurate volume measurements using volumetric glassware. By leveraging gravimetric dosing mixing systems like Quantos powder, this approach minimizes variability, reduces OOS results, and streamlines laboratory processes.
The Impact of Out-of-Specification Results
OOS results have long challenged the pharmaceutical industry, notably since a 1993 court ruling involving Barr Labs, which clarified that OOS results may stem from laboratory errors rather than batch failures. The FDA’s 2006 guidelines emphasized thorough laboratory investigations to identify root causes. Figure 1 highlights sample processing and human error as the primary sources of OOS results, with sample processing consuming over 61% of laboratory time. Figure 2 illustrates the formal OOS investigation process, which can take days or months, costing thousands of dollars and generating complex Corrective and Preventative Actions (CAPAs) that complicate SOPs.
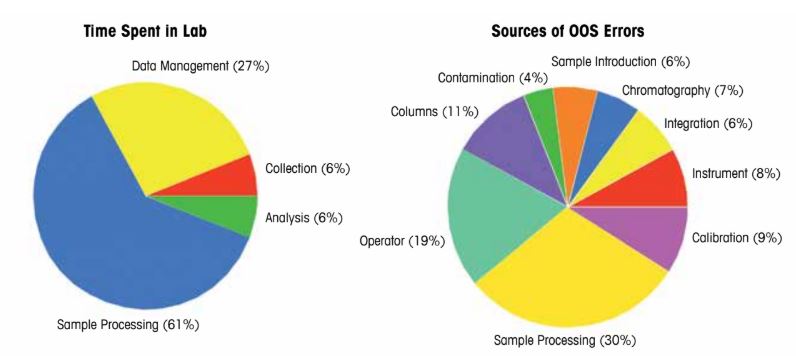
Figure 1: Sources of OOS results and time spent in laboratory
Figure 2: illustrates the accepted formal process for how to investigate OOS results

Figure: 2 Formal process for an OOS investigation.
Good Weighing Practice: Ensuring Accuracy
The Good Weighing Practice (GWP) standard provides a scientific foundation for weighing accuracy in labs, addressing measurement uncertainty and minimum weight requirements per USP General Chapter <41>. Figure 3 shows how measurement uncertainty increases with smaller sample weights, emphasizing the need for precise calibration balance procedures. A common misconception is that tare weight contributes to minimum weight compliance, but USP General Chapter <1251> clarifies that only the sample weight matters.

Figure 3: Measurement Uncertainty: Absolute (green line) and relative (blue line) measurement uncertainty of a weighing instrument. The accuracy limit of the balance, the so-called minimum weight, is the intersection point between relative measurement uncertainty and the required weighing accuracy.
To account for types of laboratory variability, such as environmental factors and operator inconsistencies, a safety factor is applied—typically 2 for manual weighing but reduced to 1.5 for automated sample preparation systems like Quantos. Figure 4 likely illustrates the application of this safety factor, showing how it compensates for variability in weighing processes to ensure reliable results.

Figure 4: Safety Factor: Variability of the relative measurement uncertainty due to changing environmental conditions and influences introduced by the operator. Weighing in the green area guarantees adherence to the weighing accuracy requirements (application of a safety factor).
Challenges with Volumetric Methods
The volumetric method relies on volumetric glassware, introducing several error sources:
- Failure Rates: Up to 50% of new glassware fails Class A specifications, per Coleman and Harris (2006).
- alibration Temperature: Flasks are calibrated at 20°C, and deviations from endothermic/exothermic reactions or sonication introduce errors.
- Cross-Contamination Risk: Reusing glassware requires rinsing, increasing solvent waste and contamination risks.
- Tolerances: Table 1 shows higher relative percent errors for smaller glassware sizes.
Figure 5 details a typical volumetric method workflow, outlining steps like gathering equipment and material, weighing, labeling, sonicating, and cleaning. This multi-step process, often exceeding 40 steps for multiple samples, is prone to laboratory errors. Figure 6 contrasts tuned and untuned sonicators, highlighting inconsistent energy distribution in mixing, a key source of variability. Manual labeling further risksNums OOS results due to identification errors, requiring solvent-based removal that adds inefficiencies.
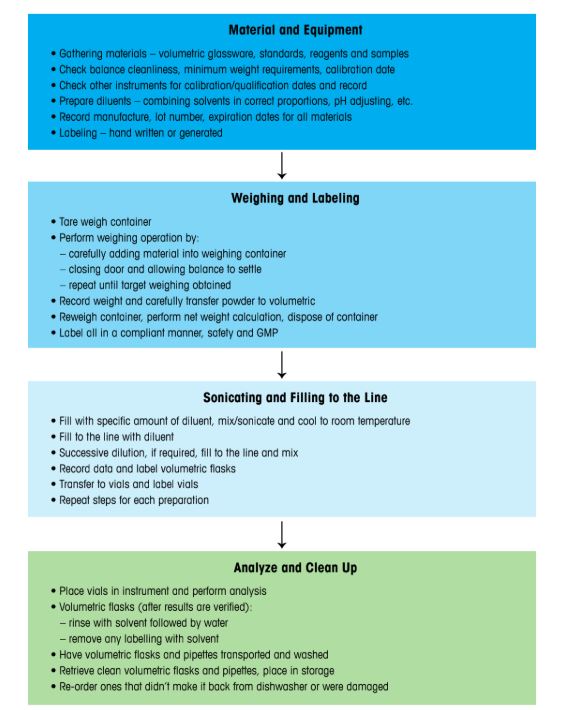
Figure 5: Simple sample preparation workflow
| Pipettes | Flasks | ||
|---|---|---|---|
| Volume (mL) | Relative % Error | Volume (mL) | Relative % Error |
| 1 | 0.60 | 5 | 0.40 |
| 2 | 0.30 | 10 | 0.20 |
| 3 | 0.33 | 25 | 0.12 |
| 4 | 0.25 | 50 | 0.10 |
| 5 | 0.20 | 100 | 0.08 |
| 10 | 0.20 | 200 | 0.05 |
Table 1: Relative percent errors for Class A glassware

Figure 6: Foils from a tuned and untuned sonicator
The Advantages of Gravimetric Sample Preparation
Gravimetric sample preparation, as per USP General Chapter <1251>, uses automated sample preparation systems like the Quantos powder gravimetric dosing mixing system, depicted in Figure 7 . These systems, equipped with RFID (radio frequency identification) for process security, weigh solids and liquids into disposable vials, eliminating cross-contamination risk. volumetric glassware and reducing

Figure 7: Gravimetric dispensing systems: XPE balances with Quantos powder and liquid dosing modules
Key Benefits of Gravimetric Sample Preparation
- Improved Accuracy: Weighing solids and liquids ensures precise concentrations, eliminating meniscus reading errors.
- Process Efficiency: Figure 8 shows a gravimetric workflow with 30% fewer steps than the volumetric method, saving time and reducing error-prone steps.
- Reduced Sample Volume: Figure 9 compares solvent usage, showing gravimetric systems use up to 79% less substance and solvent due to lower minimum weights and a reduced safety factor (e.g., 0.3 mg on an XPE205DR vs. 50 mg manually). Table 3 quantifies these savings, with automated systems like XPE205DR-PL achieving significant reductions.
- Enhanced Data Management: Automated systems record data and print labels, minimizing identification errors.
- Environmental Benefits: Smaller sample sizes reduce solvent waste, promoting sustainability.
| Sample Preparation | Volumetric | Gravimetric | ||
|---|---|---|---|---|
| Configuration |
|
|
|
|
| Sample dispensed | Manual (with spatula) |
Manual (with spatula) |
Automated or manual* | Automated or manual* |
| Calculation of amount of diluent required |
Manual | Automatic (based on actual sample weight) |
Automatic (based on actual sample weight) |
Automatic (based on actual sample weight) |
| Diluent dispensed | Manual (with pipette) | Automated | Automated | Automated |
| Diluent units | mL | g | g | g |
| Concentration units | mg/mL | mg/g | mg/g | mg/g |
| USP Minimum Weight** (0.10%, k = 2, 5% load) |
14 mg | 14 mg | 10 mg (automated) 14 mg (manual) |
7 mg (automated) 10 mg (manual) |
| Minimum Weight** (U=1.0%, k = 2, 5% load) |
1.4 mg | 1.4 mg | 1.0 mg (automated) 1.4 mg (manual) |
0.7 mg (automated) 1.0 mg (manual) |
* = Automated powder dispensing for free-flowing powders; Manual dispensing for sticky powders, pastes, gels, liquid samples, etc.
** = typical value
Table 2: Gravimetric dispensing system specifications
These automated gravimetric systems are being adopted by analytical and QA/QC laboratories in the pharmaceutical industry.
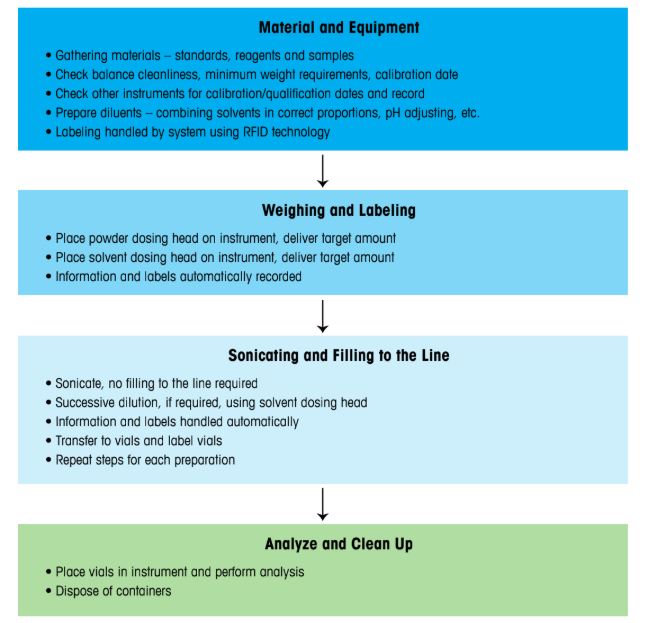
Figure 8: New simplified sample prep workflow using a gravimetric approach

Figure 9: Comparison of volumetric and gravimetric sample preparation on the amount of solvent used
| Sample Preparation | Manual Volumetric | Automatic Gravimetric | ||||||
|---|---|---|---|---|---|---|---|---|
| Configuration |
|
|
|
|
||||
| USP Minimum Weight* (0.10%, k = 2, 5% load) |
14 mg | 14 mg | 10 mg (automated) | 7 mg (automated) | ||||
| Recommended safety factor ** |
2 | 2 | 1.5 | 1.5 | ||||
| Lowest sample weighed out |
28 mg | 28 mg | 13.5 mg | 10.5 mg | ||||
| Concentration prepared examples (mg/mL or mg/g) | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 |
| Amount of solid dis- pensed (mg) |
50 | 50 | 28 | 28 | 15 | 15 | 10.5 | 10.5 |
| Amount of diluent required (mL or g) |
100 | 50 | 56 | 28 | 30 | 15 | 21 | 10.5 |
| Substance savings compared to Manual method: | 44% | 70% | 79% | |||||
* = typical value
** = The safety factor quoted is that recommended for stable environments and trained operators. For unstable environmental conditions
or insufficiently trained operators higher safety factors should be used.
Table 3: Substance saving based on automated sample preparation methods
Volumetric vs. Gravimetric: A Direct Comparison
Table 4 highlights how gravimetric sample preparation reduces determinate and indeterminate errors compared to the volumetric method. For a 0.25 mg/mL solution, the volumetric method requires a 200 mL flask and 50 mg of material, while the gravimetric method uses significantly less. Table 5 details an experiment where a 0.603 mg/g standard was prepared with high precision using Quantos, contrasted with Table 6, showing higher variability (0.60% RSD) in the volumetric method versus 0.40% for the gravimetric method. Table 7 summarizes these advantages, emphasizing substance savings, reduced cross-contamination risk, and improved accuracy.
| Step | Volumetric | Gravimetric | ||
|---|---|---|---|---|
| Determinate Errors | Indeterminate Errors | Determinate Errors | Indeterminate Errors | |
| 200 mL container | 0.05% | Uncalibrated | N/A – volumetric flasks are not required | |
| Weigh 50 mg sample | – | 0.1% balance Others are accounted for using a safety factor of 2 or higher |
– | 0.1% balance Others are accounted for using a safety factor of 1.5, if automated (in a controlled lab environment) |
| Sample transfer | Re-weighing container | Powder transfer | N/A – transfer of sample to volumetric flask is not required |
|
| Fill to mark | – | Reading meniscus and temperature effects |
N/A – no subjective reading of meniscus required with gravimetric method |
|
Table 4 : Comparing errors in volumetric and gravimetric processes
| # Solution | Dose API (mg) |
Solvent added (g) |
Solution Concentration (mg/g) |
Area | Area correlated to 0.6 mg/g |
Injector Accuracy Solution 1 |
Area |
|---|---|---|---|---|---|---|---|
| 1 | 10.105 | 16.7481 | 0.60299 | 2596.8833 | 2584.00634 | Inj. 1 | 2593.06665 |
| 2 | 10.320 | 17.1048 | 0.60298 | 2595.35474 | 2582.52818 | Inj. 2 | 2604.43384 |
| 3 | 10.140 | 16.8063 | 0.60298 | 2597.53027 | 2584.69296 | Inj. 3 | 2604.12378 |
| 4 | 10.125 | 16.7815 | 0.60298 | 2595.5564 | 2582.72885 | Inj. 4 | 2604.89429 |
| 5 | 10.250 | 16.9885 | 0.60299 | 2597.12427 | 2584.24611 | Inj. 5 | 2607.99683 |
| 6 | 10.200 | 16.9058 | 0.60298 | 2596.04517 | 2583.2152 | Inj. 6 | 2605.37231 |
| 7 | 10.130 | 16.7895 | 0.60299 | 2602.42212 | 2589.51769 | Inj. 7 | 2602.29541 |
| 8 | 10.040 | 16.6408 | 0.60297 | 2609.64868 | 2596.79455 | Inj. 8 | 2608.33862 |
| 9 | 10.275 | 17.0297 | 0.60299 | 2604.74341 | 2591.82747 | Inj. 9 | 2593.23218 |
| – | – | – | – | – | – | Inj. 10 | 2601.73145 |
| Mean | 10.176 | 16.866 | 0.603 | 2599.479 | 2586.617 | Mean | 2602.549 |
| σ | 0.09092503 | 0.150659114 | 7.07107e-06 | 5.000822667 | 4.983629696 | σ | 5.37642343 |
| RSD (%) | 0.894 | 0.893 | 0.001 | 0.19 | 0.19 | RSD (%) | 0.21 |
Table 5: Automated gravimetric sample preparation data
| Manual Dose | |||
|---|---|---|---|
| # | Blend dosed mg |
Flask Size ml |
Conc. achieved mg/ml |
| 1 | 12.920 | 20 | 0.646 |
| 2 | 12.960 | 20 | 0.648 |
| 3 | 12.860 | 20 | 0.643 |
| 4 | 12.840 | 20 | 0.642 |
| 5 | 13.060 | 20 | 0.653 |
| 6 | 12.980 | 20 | 0.649 |
| 7 | 12.860 | 20 | 0.643 |
| 8 | 12.800 | 20 | 0.640 |
| 9 | 12.860 | 20 | 0.643 |
| 10 | 12.920 | 20 | 0.646 |
| Mean | 12.906 | 20 | 0.645 |
| σ | 0.078 | 0.0 | 0.004 |
| % RSD | 0.60% | 0.0 | 0.57% |
Table 6: Manual (volumetric) sample preparation
| Manual Volumetric | Automated Gravimetric | |
|---|---|---|
| Substance saving | Uses for more substance than necessary due to: – Higher minimum weight – Higher safety factor – Scaling up to appropriate size of volumetric flask |
– Smaller minimum weight = smaller sample sizes – Dispense directly into target vessel and avoid loss on transfer – No sample spillage or overshooting target weight – Eliminates need to prepare duplicate samples due to OOS weighing errors |
| Diluent addition accuracy | Manual volumetric method has many potential error risks: – Meniscus reading is subjective – Temperature deviation of contents compared to calibrated temperature – Cross-contamination risk – Incorrect pour/drain times – Dishwasher damage – Up to 50% failure rates to comply with Class A specifications – Recalibration recommended every 10 years |
Automated gravimetric method is highly accurate: – Amount of liquid needed is calcu- lated automatically based on the actual weight of solid which. – Eliminates manual calculation errors – Eliminates human error in choice of pipette – Eliminates human error in choice of flask – The amount of solvent dispensed gravimetrically is far above the mini- mum weight therefore measurement uncertainty of this step is negligible |
| Manual Volumetric | Automated Gravimetric | |
|---|---|---|
| Process and data security | – Requires hand transcription – Relies on diligence of analyst |
– Automated data transcription – Labels/documentation generated automatically – Integrated RFID chip stores and tracks substance information and eliminates risk of sample confusion – Data is electronically recorded and fully traceable |
| Potential for cross-contamination | Volumetric flasks are re-used. They have to be rinsed before and after each use. | – Disposable dosing head is used for a single substance only which elimi- nates cross contamination – The dosing head is stored in a trans- port container which protects it from the atmosphere – Each solvent has a unique liquid dosing head. There are no valves or washing of lines is necessary. – Disposable vial used to prepare samples. |
| User Safety | Manual weighing of solids and sample preparation involves close contact for user. | Automated dispensing reduces user exposure:– The powder is contained in the dosing head– The dosing head is a closed system – Only the required amount of powder is released from the dosing head – An optional safety enclosure is avail- able |
Table 7: Comparison of volumetric vs. gravimetric methods
Conclusion
Gravimetric sample preparation revolutionizes analytical laboratory workflows by addressing the root causes of OOS results. Using automated sample preparation systems like Quantos, laboratories achieve superior weighing accuracy, eliminate volumetric glassware errors, and enhance efficiency. These systems reduce laboratory errors, minimize waste, and improve compliance through automated data management. For laboratories seeking precision and reliability, the gravimetric method is a critical advancement.
Inkarp Instruments, a trusted supplier and service provider for Mettler Toledo products in India, offers cutting-edge scientific solutions customized to meet the evolving demands of modern research. Committed to excellence and dependability, we equip researchers nationwide with innovative technology and expert support to drive advancements and achieve groundbreaking discoveries.
References:
1. United States of America v. Barr Laboratories, Inc., 812 F Supp 458 (DNJ 1993).
2. U.S. Food and Drug Administration. Guidance for Industry: Investigating out-of-specification (OOS) test results for pharmaceutical production. FDA. Available at: www.fda.gov/downloads/Drugs/GuidanceCompli-anceRegulatoryInformation/Guida nces/UCM070287.pdf.
3. Majors, R.E. LC/GC Magazine, 1991, 1997, 2002
4. General Chapter <41> “Weights and Balances”, US Pharmacopeia USP34 – NF29, Rockville, Maryland, 2013, Online-Edition
5. GWP® - Good Weighing PracticeTM – A Risk-Based Approach to Select and Test Weighing Instruments, White Paper, Mettler-Toledo AG, Greifensee, Switzerland, July 2009.
6. Reichmuth A., Fritsch K., Good Weighing Practices in the Pharmaceutical Industry – Risk-Based Qualifica- tion and Life Cycle Management of Weighing Systems, Pharmaceutical Engineering, Volume 29, Number 6, ISPE, Tampa FL, USA, 2009.
7. Fritsch K., Quenot J.-L., Good Weighing Practices – Avoid OOS Results with Proper Weighing, Pharmaceuti- cal Formulation & Quality, Volume 14, Number 1, Wiley-Blackwell, Hoboken, NJ, USA, 2012.
8. Evaluation of Measurement Data – Guide to the Expression of Uncertainty in Measurement (GUM), JCGM 100:2008, Bureau International des Poids et Mesures, Sèvres, France, 2008. Available at www.bipm.org.

